-
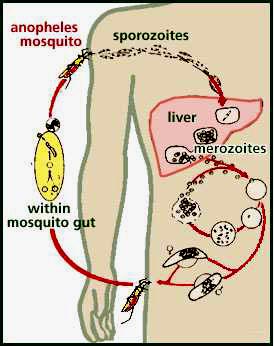
paludisme et drépanocytose
la drépanocytose, qui est donc en générale délétère, a un effet protecteur diminuant la sévérité du paludisme. A priori, pour faire simpliste, l'hématozoaire du paludisme déteste les globules rouges en forme de faucille. Il ne peut donc pas infester un patient drépanocytaire de manière massive, ce qui diminue assez nettement le risque de décès
http://grangeblanche.hautetfort.com/archive/2006/02/21/paludisme-et-drepanocytose.html
While in the red blood cell, the malarial parasite induces N and S hemoglobin to go to the deoxygenated form through a mechanism known as the Bohr effect. According to this effect, when either N or S hemoglobin is put in an acidic environment, or one high in CO2, they tend to release oxygen, and convert to the deoxygenated form. The malarial parasite metabolizes food and produces carbon dioxide as a waste product. This carbon dioxide, when in an aqueous environment like that inside a red blood cell, forms carbonic acid. Because of these high levels of C02 and acid, the hemoglobin in a parasitized red blood cell tends to be in the deoxygenated form. If a red blood cell contains S hemoglobin and a malarial parasite, the S hemoglobin will be deoxygenated, aggregate, and sickle the red blood cell.
The malarial parasite can not live in a sickled red blood cell for two reasons. First, the body sends sickled red blood cells to the spleen for elimination. The body senses that sickled red blood cells are improperly formed, so destroys as many sickled cells as possible. If a parasite is in the cell, it also is destroyed. Second, because the cell membrane of the sickled red blood cell is stretched by its unusual shape, it becomes porous. The sickled cell "leaks" nutrients, like potassium, that the parasite needs to survive, so the parasite dies. Because the malarial parasite can not live in sickled cells, an individual with S hemoglobin is therefore resistant to the malaria.
The relationship between malaria and sickle cell anemia is undeniable : both biochemical evidence and demographic evidence support the theory that sickle-cell anemia imparts malarial resistance. Biochemically, we understand that deoxygenated S hemoglobin clumps (grouper), sickling (faucille) cells. We also understand that when the malarial parasite inhabits a red blood cell, it induces the cell to sickle by the Bohr effect. This sickling of cells imparts malarial resistance to the inflicted person.
http://www.physics.ohio-state.edu/~wilkins/writing/Samples/shortmed/nelson/sickle.html
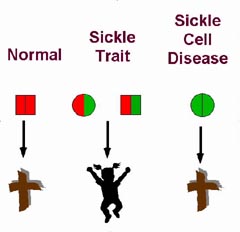 The precise mechanism by which sickle cell trait imparts resistance to malaria is unknown. A number of factors likely are involved and contribute in varying degrees to the defense against malaria.
The precise mechanism by which sickle cell trait imparts resistance to malaria is unknown. A number of factors likely are involved and contribute in varying degrees to the defense against malaria.Red cells from people with sickle trait do not sickle to any significant degree at normal venous oxygen tension. Very low oxygen tensions will cause the cells to sickle, however. For example, extreme exercise at high altitude increases the number of sickled erythrocytes in venous blood samples from people with sickle cell trait (Martin, et al., 1989). Sickle trait red cells infected with the P. falciparum parasite deform, presumably because the parasite reduces the oxygen tension within the erythrocytes to very low levels as it carries out its metabolism. Deformation of sickle trait erythrocytes would mark these cells as abnormal and target them for destruction by phagocytes( Luzzatto, et al., 1970).
Experiments carried out in vitro with sickle trait red cells showed that under low oxygen tension, cells infected with P. falciparum parasites sickle much more readily than do uninfected cells (Roth Jr., et al., 1978). Since sickle cells are removed from the circulation and destroyed in the reticuloendothelial system, selective sickling of infected sickle trait red cells would reduce the parasite burden in people with sickle trait. These people would be more likely to survive acute malarial infections.
Other investigations suggest that malaria parasites could be damaged or killed directly in sickle trait red cells. P. falciparum parasites cultured in sickle trait red cells died when the cells were incubated at low oxygen tension (Friedman, 1978). In contrast, parasite health and growth were unimpeded in cells maintained at normal atmospheric oxygen tensions. The sickling process that occurs at low oxygen tensions was presumed to harm the parasite in some fashion. Ultrastructural studies showed extensive vacuole formation in P. falciparum parasites inhabiting sickle trait red cells that were incubated at low oxygen tension, suggesting metabolic damage to the parasites (Friedman, 1979). Prolonged states of hypoxia are not physiological, raising questions about degree to which these data can be extrapolated to human beings. However, they do suggest mechanisms by which sickle hemoglobin at the concentrations seen with sickle cell trait red cells could impair parasite proliferation.
Other investigations suggest that oxygen radical formation in sickle trait erythrocytes retards growth and even kills the P. falciparum parasite (Anastasi, 1984). Sickle trait red cells produce higher levels of the superoxide anion (O2-) and hydrogen peroxide (H2O2) than do normal erythrocytes. Each compound is toxic to a number of pathogens, including malarial parasites. Homozygous hemoglobin S red cells produce membrane associated hemin secondary to repeated formation of sickle hemoglobin polymers. This membrane-associated hemin can oxidize membrane lipids and proteins (Rank, et al., 1985). Sickle trait red cells normally produce little in the way of such products. If the infected sickle trait red cells form sickle polymer due to the low oxygen tension produced by parasite metabolism, the cells might generate enough hemin to damage the parasites (Orjih, et al., 1985).
The immune system is key to weathering attacks by P. falciparum. Maternal antibodies passed to newborns prior to birth provide some protection from malaria for the first several months of life. Thereafter, the onus is on the toddler's immune system to provide the needed defense. Epidemiological studies performed in regions with endemic malaria show that antibody titers to P. falciparum are lower in children with sickle cell trait than in children with genes only for hemoglobin A (Cornille-Brogger, et al., 1979). The investigators speculated that lower levels of immune activation might reflect a lower parasite burden in children with sickle cell trait due to clearance of the infected red cells. Analysis of people with sickle cell trait and people homozygous for hemoglobin A in the regions with endemic malaria in fact show a lower mean parasite burden in people with sickle cell trait relative to hemoglobin A homozygotes (Fleming, et al., 1979). In contrast, children with sickle cell disease have a high fatality rate, with acute malarial infections being a chief cause of death (Fleming, 1989).
Hemoglobin C is also believed to protect against malaria, although data on this point were not conclusive until recently. Hemoglobin C lacks the in vitro antimalarial activity of hemoglobin S. Some epidemiological studies found no evidence for protection against malaria in people with either homozygous or heterozygous hemoglobin C (Willcox, et al., 1983). The relatively small number of patients with hemoglobin C in these studies left the conclusions open to question, however.
The issue was finally settled in an investigation that included more than 4,000 subjects (Modiano, et al., 2001). Hemoglobin C heterozygotes had significantly fewer episodes of P. falciparum malaria than did controls with only hemoglobin A. The risk of malaria was lower still in subjects who were homozygous for hemoglobin C. Homozygous hemoglobin C produces a mild hemolytic anemia and splenomegaly. The much milder phenotype of the condition relative to homozygous hemoglobin S led the investigators to speculate that without medical intervention for malaria, hemoglobin C would replace hemoglobin S the over the next few thousand years as the dominant "antimalarial" hemoglobin in West Africa.
The thalassemias also reached levels of expression in human populations by protecting against malaria. The inbalance in globin chain production characteristic of thalassemia produces membrane oxidation by hemichromes and other molecules that generate reactive oxygen species (Grinberg, et al., 1995;Sorensen, et al., 1990). Reactive oxygen species also injure and kill malaria parasites (Clark, et al., 1989).
In vitro malaria toxicity of thalassemic red cells is most easily seen in cells containing hemoglobin H (ß-globin tetramers) (Ifediba, et al., 1985; Yathavong, et al., 1988). Hemoglobin H occurs most often in people with three-gene deletion alpha-thalassemia (Zhu, et al., 1993). The compound heterozygous condition of two-gene deletion alpha thalassemia and hemoglobin Constant Spring also produces erythrocytes that contain hemoglobin H (Derry, et al., 1988). Two gene deletion alpha thalassemia also protects the host from malaria, however. The process is difficult to demonstrate with in vitro cultures of malaria parasites. Alpha thalassemia may protect against malaria in part by altering the immunue response to parasitized red cells (Luzzi, et al., 1991) In any event, epidemiological studies show clear evidence of protection provided by two-gene deletion alpha thalassemia (Flint, et al., 1986; Modiano, et al., 1991).
One of the key reasons for the high fatality rate in P. falciparum malaria is the occurence of so-called cerebral malaria. Patients become confused, disoriented and often lapse into a terminal coma. Clumps of malaria-infested red cells adhere to the endothelium and occlude the microcirculation of the brain with deadly consequences. The P. falciparum parasite alters the characteristics of the red cell membrane, making them more "sticky". Clusters of parasitized red cells exceed the size of the capillary circulation blocking blood flow and producing cerebral hypoxia. Thalassemic erythrocytes adhere to parasitized red cells much less readily than do their normal counterparts (Carlson, et al., 1994). This alteration would lessen the chance of developing cerebral malaria.
The rise to high frequency of alleles that produce red cells deficient in glucose-6-phosphate dehydrogenase activity is one of the most dramatic examples of the selective pressure of malaria on humankind (Ruwende, et al., 1995; Tishkoff, et al., 2001). Reactive oxygen species are formed continually as erythrocytes take up oxygen from the lungs and release it to the preriperal tissues. As noted above, malaria parasites are easily damaged by these reactive oxygen species (Friedman, 1979). Glucose-6-phosphate dehydrogenase prevents oxidation of the heme group. In its absence, hemichromes and other species that generate reactive oxygen species accumulate in erythrocytes (Janney, et al., 1986). P. falciparum grow poorly in erythrocytes that are deficient in G-6-PD (Roth JR, et al., 1983). Malaria continues to battle back in this struggle, however. The advent of P. falciparum parasites that produce their own G-6-PD provides ample evidence of the continuing moves and counter-moves in the battle between man and malaria (Usanga, et al, 1985).
http://sickle.bwh.harvard.edu/malaria_sickle.html
-
Commentaires
Mon petit cahier de sciences naturelles

